
The Good, Bad, and Unseen Impact of UV Rays on Life and Our Planet

UV Rays may be bad for your skin - but they are good for water quality? Can we see, smell, taste UV? Are there any interesting facts about what happens to UV when it passes through other atmosphere’s other than Earth’s? Is it important for life on Earth and at what levels? Are there any things that humans are doing that are affecting the amount of UV penetrating the atmosphere? What ever happened to that Ozone hole?
UV, like light, infrared radiation, x-rays, microwaves, gamma radiation, and radio waves are all part of the electromagnetic spectrum. The difference between them is what their wavelengths are and how much energy their photons carry – the shorter the wavelength, the greater the energy. UV is just beyond visible light on the electromagnetic spectrum and has a shorter wavelength which corresponds to a higher energy.
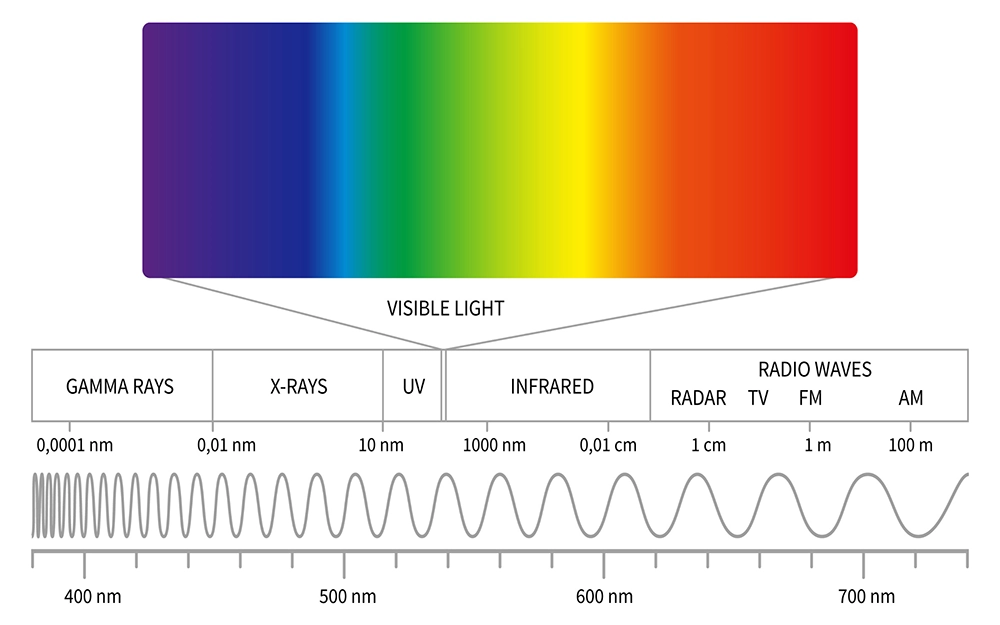
Like light, UV includes a range of wavelengths and energies. Visible light has a wavelength of from 700 (red light) to 380 (violet light) nanometers (one billionth of a meter). UV, by its very name, ultraviolet, begins just beyond violet light at ~ 400 nm (nanometers) to 100 nm at which point x-rays begin. UV is usually subdivided into three smaller ranges: UVA (400-315 nm), UVB (315-280 nm), and UVC (280-100 nm).
That part of the electromagnetic spectrum beyond visible light, which includes UV, x-rays, and gamma radiation, carries enough energy to break chemical bonds and produce ions which is why that kind of radiation is known as ionizing (it produces ions) radiation. Note that microwave radiation with a far longer wavelength than even visible light, is not ionizing radiation. It can fry you but will not ionize anything.
Under most conditions, visible light is just a little short of having enough energy to ionize anything but with the right catalysts and some other help, it can do it which is why solar cells can create electricity using visible light and plants can photosynthesize.
UVA has enough energy to produce a tan in humans but it does damage and age the skin. The more energetic UVB can penetrate the skin and break chemical bonds to create vitamin D3 which is a good thing but, unfortunately, it isn’t limited to breaking chemical bonds that create vitamin D3 but it can and does disrupt other important molecules. Prolonged exposure to too much UV can gradually destroy the folic acid stores of the body to produce a condition known as tropical sprue with potentially dire consequences.
Skin color is tightly linked to UV exposure. You need enough UV exposure to produce vitamin D3 which is critical in bone growth but not so much that it reduces the folic acid stores. People whose ancestors were in the high-UV tropics, tend to have darker skin because the greater amounts of melanin in the skin protect the body by absorbing/blocking some of the UV.
Move those same people far away from the tropics to low UV areas, and they become susceptible to rickets because they cannot produce enough vitamin D3 which is why, in many countries, milk is fortified with vitamin D. People with a light complexion have much less problem with getting enough UV exposure to manufacture vitamin D, except, perhaps, in the winter. Move them to the tropics and they have to worry about severe folic acid deficiency and skin cancer. So, UV can be bad for skin but a certain amount of exposure is also necessary for good health. Keeping a good balance can be a problem.
Human beings cannot see UV although some birds and insects like bees can. A UV lamp produces what is sometimes called black light (because we cannot see it) but when absorbed by certain substances will create a fluorescence or phosphorescence (it continues to shine for a while after the power is shut off) as the absorbed UV is re-emitted as visible light. A fluorescent light actually emits UV but the white powder in the inside of the fluorescent tube absorbs the UV and re-emits it as white light.
Visible light has no smell or taste and neither does UV although we can all feel the result of too much UV exposure – just ask anyone who has had a sunburn. We older folks know well another consequence of a lifetime’s exposure to UV – cataracts, and maybe skin cancer.
Being ionizing radiation, UV can sterilize water, killing any microbes that might be in it which is a good thing from our point of view. A few feet of water is enough to absorb/block all of the UV which is what protected marine life of the early Earth when there was no ozone layer to block most of the UV.
For most of Earth’s history, there was not enough oxygen in the atmosphere to create a protective ozone layer in the stratosphere. Trace amounts of oxygen began to appear some 3 billion years ago but even half a billion years ago the amount of atmospheric oxygen was only a percent or two. Compare that to the current 21%.
There is evidence that there was life in the Earth’s oceans as long ago as 4 billion years but land plants did not begin to appear until only ~ 470 million years ago. Why did it take so long? It took billions of years for marine photosynthesis to build up the atmosphere level of oxygen high enough to create a good stratospheric ozone layer. Without that ozone layer, the UV levels at the surface of the Earth would have been lethal to anything sticking its head out of the water.
There is talk of colonies on the moon and Mars but the moon, with no atmosphere at all and Mars with a very thin atmosphere and no ozone layer have very high UV levels at their surfaces. Any lunar of Martian colonies will have to go underground for protection from UV (and cosmic radiation).
UV, being an ionizing radiation, is good at breaking chemical bonds which, in living things, can cause cancer and mutations and even death. It can also break the chemical bonds of atmospheric compounds, especially those with hydrogen bonds, in a process known as photolysis (literally, to break/cleave with light). Common simple hydrogen compounds in some planetary atmospheres vulnerable to photolysis include water (H2O), methane (CH4), and ammonia (NH3).
The bonds are stronger for something like carbon dioxide (CO2) which the UV cannot split. A very early Venus probably had oceans like the Earth but being closer to the sun, a hotter Venus quickly ended up with oceans of water vapor in its atmosphere. Water vapor, like carbon dioxide, is a greenhouse gas and so all of that Venusian water vapor made Venus even hotter than it is today. It also made the water vulnerable to photolysis which split the water molecule into hydrogen and oxygen gas.
Venus isn’t massive enough to hold onto hydrogen gas (neither is the Earth) and so the hydrogen was lost to space. The oxygen combined with the very hot surface rock to form oxides, giving the surface of Venus a reddish hue which you can’t see because of the very thick clouds. Mars, too, likely lost some water vapor to photolysis although, being much colder, most of the water froze out on the surface.
The Earth, too, lost substantial water to photolysis before the formation of an ozone layer but being cooler than Venus, kept most of it in surface oceans rather than in the air as vulnerable water vapor.
Normal gaseous oxygen is diatomic (two oxygen atoms bonded together, O2). The ozone molecule is a triplet, O3. The UV energy is absorbed by the ozone molecule by splitting the ozone: O3 + UV ––> O2 + O. That single oxygen atom is very reactive and, aided by a nitrogen molecule (N2) or another O2, will quickly form ozone again: O + O2 ––> O3. And then humans entered the picture.
Humans have been very good at creating chemicals and technologies that improve our lives, things such as refrigerators and air conditioning. These cooling devices require some substance with a high heat capacity that can be easily changed from a gas to a liquid and back, in the process transferring heat. Early refrigerators sometimes used compounds like ammonia and sulfur dioxide which worked well but, if there were a leak, could be quite toxic. And then, in 1928, scientists developed freon.
Unlike previous refrigerants, freon is odorless and not toxic. It is actually a group of very stable compounds of carbon, chlorine, and fluorine, collectively known as CFCs (chlorofluorocarbons), such as freon 22 which is CCl2F2. Inevitably, during production and use, some freon escaped into the atmosphere. Some of it reached the ozone layer with the following results:
You can see that the UV is energetic enough to split a chlorine atom off of the molecule in step one. Like a single oxygen atom, a single chlorine atom is very reactive, enough so that it pulls an oxygen off of an ozone molecule in step two destroying the ozone molecule. The UV then splits the ClO, leaving behind a very reactive single oxygen atom which yanks an oxygen off of another ozone atom, destroying that ozone in step four. Also left behind is a very reactive single chlorine atom which then starts the cycle over and over again, destroying thousands of ozone molecules.
The chemists tried to make refrigerants less hostile to ozone, such as the HFCs (hydrofluorocarbons) which proved to be quite benign to ozone but another problem arose. It turns out that HFCs are super greenhouse gases, thousands of times more potent than carbon dioxide. Sometimes you just can’t win; and so the search continues.
Meanwhile, world production of freon has pretty much halted. However, freon has an atmospheric half-life of something on the order of years to decades. It also happens that the very cold temperatures in the stratosphere above the polar areas enhance the destruction of ozone which is why the ozone hole(s) appear above the polar areas. As the amount of stratospheric freon slowly diminishes, the ozone holes slowly close.
To explore more water science related content, see Professor Dr. Redmond's section on Water Science Basics and Water in the Universe.



